The absorption and subsequent re-radiation of light by organic and inorganic specimens is typically the result of well-established physical phenomena described as being either fluorescence or phosphorescence. The emission of light through the fluorescence process is nearly simultaneous with the absorption of the excitation light due to a relatively short time delay between photon absorption and emission, ranging usually less than a microsecond in duration. When emission persists longer after the excitation light has been extinguished, the phenomenon is referred to as phosphorescence.
British scientist Sir George G. Stokes first described fluorescence in 1852 and was responsible for coining the term when he observed that the mineral fluorspar emitted red light when it was illuminated by ultraviolet excitation. Stokes noted that fluorescence emission always occurred at a longer wavelength than that of the excitation light. Early investigations in the 19th century showed that many specimens (including minerals, crystals, resins, crude drugs, butter, chlorophyll, vitamins, and inorganic compounds) fluoresce when irradiated with ultraviolet light. However, it was not until the 1930s that the use of fluorochromes was initiated in biological investigations to stain tissue components, bacteria, and other pathogens. Several of these stains were highly specific and stimulated the development of the fluorescence microscope.
The technique of fluorescence microscopy has become an essential tool in biology and the biomedical sciences, as well as in materials science due to attributes that are not readily available in other contrast modes with traditional optical microscopy. The application of an array of fluorochromes has made it possible to identify cells and sub-microscopic cellular components with a high degree of specificity amid non-fluorescing material. In fact, the fluorescence microscope is capable of revealing the presence of a single molecule. Through the use of multiple fluorescence labeling, different probes can simultaneously identify several target molecules simultaneously. Although the fluorescence microscope cannot provide spatial resolution below the diffraction limit of specific specimen features, the detection of fluorescing molecules below such limits is readily achieved.
A variety of specimens exhibit autofluorescence (without the application of fluorochromes) when they are irradiated, a phenomenon that has been thoroughly exploited in the fields of botany, petrology, and the semiconductor industry. In contrast, the study of animal tissues and pathogens is often complicated with either extremely faint or bright, nonspecific autofluorescence. Of far greater value for the latter studies are added fluorochromes (also termed fluorophores), which are excited by specific wavelengths of irradiating light and emit light of defined and useful intensity. Fluorochromes are stains that attach themselves to visible or sub-visible structures, are often highly specific in their attachment targeting, and have a significant quantum yield (the ratio of photon absorption to emission). The widespread growth in the utilization of fluorescence microscopy is closely linked to the development of new synthetic and naturally occurring fluorophores with known intensity profiles of excitation and emission, along with well-understood biological targets.
Fundamentals of Excitation and Emission
The basic function of a fluorescence microscope is to irradiate the specimen with a desired and specific band of wavelengths, and then to separate the much weaker emitted fluorescence from the excitation light. In a properly configured microscope, only the emission light should reach the eye or detector so that the resulting fluorescent structures are superimposed with high contrast against a very dark (or black) background. The limits of detection are generally governed by the darkness of the background, and the excitation light is typically several hundred thousand to a million times brighter than the emitted fluorescence.
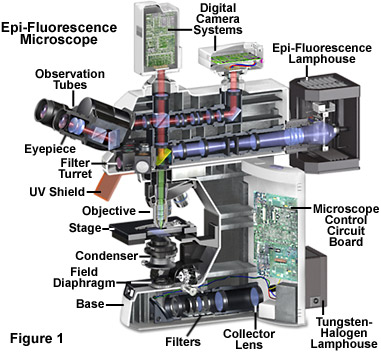
Illustrated in Figure 1 is a cutaway diagram of a modern epi-fluorescence microscope equipped for both transmitted and reflected fluorescence microscopy. The vertical illuminator in the center of the diagram has the light source positioned at one end (labeled the episcopic lamphouse) and the filter cube turret at the other. The design consists of a basic reflected light microscope in which the wavelength of the reflected light is longer than that of the excitation. Johan S. Ploem is credited with the development of the vertical illuminator for reflected light fluorescence microscopy. In a fluorescence vertical illuminator, light of a specific wavelength (or defined band of wavelengths), often in the ultraviolet, blue or green regions of the visible spectrum, is produced by passing multispectral light from an arc-discharge lamp or other source through a wavelength selective excitation filter. Wavelengths passed by the excitation filter reflect from the surface of a dichromatic (also termed a dichroic) mirror or beamsplitter, through the microscope objective to bath the specimen with intense light. If the specimen fluoresces, the emission light gathered by the objective passes back through the dichromatic mirror and is subsequently filtered by a barrier (or emission) filter, which blocks the unwanted excitation wavelengths. It is important to note that fluorescence is the only mode in optical microscopy where the specimen, subsequent to excitation, produces its own light. The emitted light re-radiates spherically in all directions, regardless of the excitation light source direction.
Epi-fluorescence illumination is the overwhelming choice of techniques in modern microscopy, and the reflected light vertical illuminator is interposed between the observation viewing tubes and the nosepiece housing the objectives. The illuminator is designed to direct light onto the specimen by first passing the excitation light through the microscope objective (which in this configuration, acts as a condenser) on the way toward the specimen, and then using that same objective to capture the emitted fluorescence. This type of illuminator has several advantages. The fluorescence microscope objective serves first as a well-corrected condenser and secondly as the image-forming light gatherer. Being a single component, the objective/condenser is always in perfect alignment. A majority of the excitation light reaching the specimen passes through without interaction and travels away from the objective, and the illuminated area is restricted to that which is observed through the eyepieces (in most cases). Unlike the situation in some contrast enhancing techniques, the full numerical aperture of the objective is available when the microscope is properly configured for Köhler illumination. Finally, it is possible to combine with or alternate between reflected light fluorescence and transmitted light observation and the capture of digital images.
As presented in Figure 1, the reflected light vertical illuminator comprises an arc-discharge lamphouse at the rear end (usually a mercury or xenon burner). Excitation light travels along the illuminator perpendicular to the optical axis of the microscope, passes through collector lenses and a variable, centerable aperture diaphragm, and then through a variable, centerable field diaphragm (see Figure 1). The light then impinges upon the excitation filter where selection of the desired band and blockage of unwanted wavelength occurs. The selected wavelengths, after passing through the excitation filter, reach the dichromatic beamsplitting mirror, which is a specialized interference filter that efficiently reflects shorter wavelength light and efficiently passes longer wavelength light. The dichromatic beamsplitter is tilted at a 45-degree angle with respect to the incoming excitation light and reflects this illumination at a 90-degree angle directly through the objective optical system and onto the specimen. Fluorescence emission produced by the illuminated specimen is gathered by the objective, now serving in its usual image-forming function. Because the emitted light consists of longer wavelengths than the excitation illumination, it is able to pass through the dichromatic mirror and upward to the observation tubes or electronic detector.
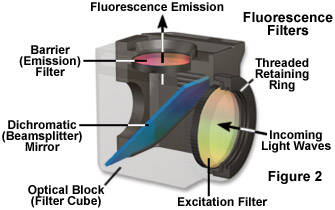
Most of the scattered excitation light reaching the dichromatic mirror is reflected back toward the light source, although a minute quantity often passes through and is absorbed by the internal coating of the mirror block. Before the emitted fluorescence can reach the eyepiece or detector, it must first pass through the barrier or suppression filter. This filter blocks (suppresses) any residual excitation light and passes the desired longer emission wavelengths. In most reflected light illuminators, the excitation filter, dichromatic mirror, and barrier filter are incorporated into an optical block (often referred to as a cube), as illustrated in Figure 2. Modern fluorescence microscopes are capable of accommodating between four and six fluorescence cubes (usually on a revolving turret or through a slider mechanism; see Figure 1) and permit the user to easily attach replacement aftermarket excitation and barrier filters, as well as dichromatic mirrors.
The vertical illuminator design should enable the user to adjust the microscope for Köhler illumination, providing a bright and even illumination aperture across the entire field of view. The corrected condensing lenses of the optical system should ensure that the image of the centerable aperture diaphragm is conjugate with the rear aperture of the focused objective. In modern illuminators, the image of the pre-focused, centerable field diaphragm is conjugate to the focused specimen and the plane of the fixed eyepiece diaphragm.
The illuminator lamphouse usually incorporates an infrared light suppression filter. The lamphouse itself should not leak harmful ultraviolet wavelengths and, preferably, should incorporate a switch to automatically shut down the lamp if the housing is inadvertently opened during operation. The lamphouse should be sturdy enough to withstand a possible burner (arc-discharge lamp) explosion during operation. In modern lamphouses, the lamp socket is equipped with adjustment knobs to permit centering the arc lamp image within the rear aperture of the objective (in Köhler illumination, these planes are conjugate). Somewhere in the light path, usually closer to the lamphouse and preceding the excitation filter, it is desirable to have a shutter in order to completely block excitation light when the specimen is not being viewed or imaged with the detector. In addition, provisions for neutral density filters should be made available (either on a wheel, turret, or slider) in order to enable the user to reduce the intensity of excitation illumination.
Stokes¡¯ Shift
Vibrational energy is lost when electrons relax from the excited state back to the ground state. As a result of the energy loss, the emission spectrum of an excited fluorophore is usually shifted to longer wavelengths when compared to the absorption or excitation spectrum (note that wavelength varies inversely to radiation energy). This well-documented phenomenon is known as Stokes¡¯ Law or Stokes¡¯ shift. As Stokes¡¯ shift values increase, it becomes easier to separate excitation from emission light through the use of fluorescence filter combinations.
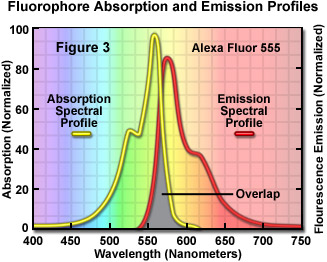
The fluorophore emission (or absorption) intensity peak is usually lower in wavelength and magnitude than that exhibited by the excitation peak, and the emission spectral profile (curve) is often a mirror image (or nearly so) of the excitation curve, but shifted to longer wavelengths, as illustrated in Figure 3 for Alexa Fluor 555, a useful probe that absorbs light in the yellow-green region and produces yellow-orange emission. In order to achieve maximum fluorescence intensity, a fluorophore (often termed a dye) is usually excited at wavelengths near or at the peak of the excitation curve, and the widest possible range of emission wavelengths that include the emission peak are selected for detection. The selection of excitation and emission wavelengths is typically based on interference filters (Figure 2). In addition, the spectral response of a microscope optical system will also depend on such factors as glass transmission efficiency (due to anti-reflection coatings), the number of lens and mirror elements, and the responsivity of the detector system.
The effective separation and detection of excitation and emission wavelengths is achieved in fluorescence microscopy through the proper selection of filters to block or pass specific wavelength bands in the ultraviolet, visible, and near-infrared spectral regions. Fluorescence vertical illuminators are designed with the purpose of controlling the excitation light through the application of readily interchangeable filter (neutral density and interference excitation balancers) insertions into the light path on the way toward the specimen, and again in the path between the specimen and the observation tubes or camera detector system. Perhaps the most important criteria, in view of relatively low fluorescence emission intensities (see discussion above), is that the light source utilized for excitation be of sufficient brightness so that the weak emission light can be maximized, and that the fluorochromes possess adequate absorption properties and emission quantum yields.
The efficiency with which a particular fluorophore absorbs a photon of the excitation light is a function of the molecular cross-section, and the likelihood of absorption is known as the extinction coefficient. Larger extinction coefficients indicate that the absorption of a photon (or quantum) in a given wavelength region is more likely. The quantum yield denotes the ratio of the number of quanta emitted compared to those absorbed (and is usually a value between 0.1 and 1.0). Quantum yield values below 1 are the result of the loss of energy through nonradiative pathways, such as heat or a photochemical reaction, rather than the re-radiative pathway of fluorescence. Extinction coefficient, quantum yield, mean luminous intensity of the light source, and fluorescence lifetime are all important factors that contribute to the intensity and utility of fluorescence emission.
Fading, Quenching, and Photobleaching
A wide spectrum of conditions often come into play that ultimately affect the re-radiation of fluorescence emission and thus reduce the intensity. The general term for a reduction of fluorescence emission intensity is fading, a catch-all category that is usually further subdivided into quenching and photobleaching phenomena for more precise descriptions. Photobleaching is the irreversible decomposition of the fluorescent molecules in the excited state because of their interaction with molecular oxygen before emission. The occurrence of photobleaching is exploited in a technique known as fluorescence recovery after photobleaching (FRAP), a very useful mechanism for investigating the diffusion and motion of biological macromolecules. The method is based upon photobleaching a sharply defined region of the specimen by an intense burst of laser light, accompanied by the subsequent observation of the rates and pattern of fluorescence recovery in the photobleached area. A related technique, known as fluorescence loss in photobleaching (FLIP), is employed to monitor the decrease of fluorescence in a defined region lying adjacent to a photobleached area. Similar to FRAP, the latter technique is useful in the investigation of molecular mobility and dynamics in living cells.

Presented in Figure 4 is a typical example of photobleaching (fading) observed in a series of digital images captured at different time points for a multiply-stained culture of Indian Muntjac deer epidermis fibroblast cells. The nuclei were stained with a bis-benzimidazole derivative (Hoechst 33258; blue fluorescence), while the mitochondria and actin cytoskeleton were stained with MitoTracker Red CMXRos (red fluorescence) and a phalloidin derivative conjugated to Alexa Fluor 488 (green fluorescence), respectively. Time points were taken in two-minute intervals using a fluorescence filter combination with bandwidths tuned to excite the three fluorophores simultaneously while also recording the combined emission signals. Note that all three fluorophores have a relatively high intensity in Figure 4(a), but the Hoechst fluorophore (blue) intensity starts to drop rapidly at two minutes and is almost completely gone at 6-8 minutes. The mitochondrial and actin stains are more resistant to photobleaching, but the intensity of both drops significantly over the course of the timed sequence (10 minutes).
The excited state relaxation process of quenching results in reduced fluorescence intensity through a variety of mechanisms involving non-radiative energy loss and frequently occurs as a result of oxidizing agents or the presence of salts or heavy metals or halogen compounds. In some cases, quenching results from the transfer of energy to another molecule (termed the acceptor), which resides physically close to the excited fluorophore (the donor), a phenomenon known as fluorescence resonance energy transfer (FRET). This particular mechanism has become the basis for a useful technique involving the study of molecular interactions and associations at distances far below the lateral resolution of the optical microscope.
Fluorescence Light Sources
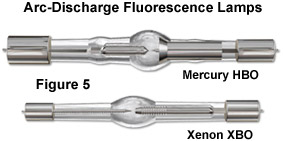
An unfortunate consequence of low emission levels in most fluorescence microscopy applications is that the number of photons that reach the eye or camera detector is also very low. In most cases, the collection efficiency of optical microscopes is less than 30 percent and the concentration of many fluorophores in the optical path ranges in the micromolar or nanomolar regions. In order to generate sufficient excitation light intensity to produce detectable emission, powerful compact light sources, such as high-energy short arc-discharge lamps, are necessary. The most common lamps are mercury burners, ranging in wattage from 50 to 200 Watts, and the xenon burners that range from 75 to 150 Watts (see Figure 5). These light sources are usually powered by an external direct current supply, furnishing enough start-up power to ignite the burner through ionization of the gaseous vapor and to keep it burning with a minimum of flicker.
The microscope arc-discharge lamp external power supply is usually equipped with a timer to track the number of hours the burner has been in operation. Arc lamps lose efficiency and are more likely to shatter if used beyond their rated lifetime (200-300 hours). The mercury burners do not provide even intensity across the spectrum from ultraviolet to infrared, and much of the intensity of the lamp is expended in the near ultraviolet. Prominent peaks of intensity occur at 313, 334, 365, 406, 435, 546, and 578 nanometers. At other wavelengths in the visible light region, the intensity is steady although not nearly so bright (but still useable in most applications). In considering illumination efficiency, mere lamp wattage is not the prime consideration. Instead, the critical parameter is the mean luminance must be considered, taking into account the source brightness, arc geometry, and the angular spread of emission.
In the past few years, optical microscopy has experienced an increase in the application of laser light sources, particularly the argon-ion and argon-krypton (ion) lasers. These lasers have the virtues of small source size, low divergence, near-monochromicity, and high mean luminance. They have become essential in scanning confocal microscopy, a technique that has proven to be a powerful tool in rendering very sharp fluorescence images through rejection of non-focused light removed from the specimen focal plane. Confocal microscopes accomplish this task through point or line scanning with coincident imaging through a conjugate aperture. Optical sections of the specimens can be stored in a host computer and reconstructed into the final image, which is then displayed on the monitor.
Filter Terminology
The common terminology applied to fluorescence microscopy filter combinations has become confusing as a result of the various initials and codes utilized by different manufacturers to identify their filters. Basically, there are three major categories of filters: excitation (often referred to as exciters), barrier (emission), and dichromatic beamsplitters (or dichroic mirrors). Fluorescence filters were formerly almost exclusively constructed from dyed glass or gelatin sandwiched between two glass plates. However, the current trend is to manufacture high-resolution filters with interference optics for excitation filters to pass or reject wavelengths of light with a great specificity and high transmission. Dichromatic beamsplitters are specialized interference filters designed to reflect or pass light of specific wavelengths when placed into the light path at a 45-degree angle (see Figures 1 and 2). Barrier filters are fabricated with both colored glass or interference coatings (or a combination of the two).
Abbreviations employed by manufacturers to identify the properties of their excitation filters include: UG (ultraviolet glass) and BG (blue glass). Shortpass filters often are denoted as KP (K is an abbreviation for kurz, which means "short" in German) or simply as SP. Several manufacturers now label their interference filters with the designation IF. Narrow band excitation interference filters are especially helpful if the Stokes¡¯ shift is small.
Acronyms or abbreviations for barrier filters include: LP or L for longpass filters, Y or GG for yellow or gelb (German) glass, R or RG for red glass, OG or O for orange glass, K for kante, a German term for edge (filter), and BA for barrier filter. When the filter type is also associated with a number, such as BA515, that designation refers to the wavelength (in nanometers) at 50-percent of its maximum transmission.
Dichromatic beamsplitters also are described by numerous abbreviations including CBS for a chromatic beam splitter, DM for dichroic mirror, TK for "teiler kante", German for edge splitter, FT for "farb teiler" (German for color splitter), and RKP for reflection short pass. All of these terms should be considered interchangeable, and modern dichromatic beamsplitters are always manufactured with interference coatings on optical glass (as opposed to organic or metallic dyes). The interference thin films are designed to produce high reflectivity for shorter wavelengths and high transmission for longer wavelengths. Dichromatic beamsplitters are oriented at a 45-degree angle to the path of the excitation light entering the optical block through the reflected light fluorescence illuminator. Their primary function is to re-direct the selected excitation (shorter) wavelengths through the objective and onto the specimen. These specialized filters also have the additional functions of passing longer wavelength fluorescence emission to the barrier filter, and reflecting any scattered excitation light back in the direction of the lamphouse.
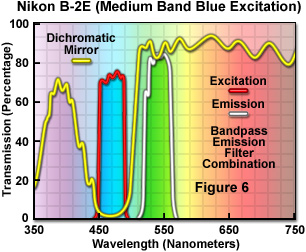
Presented in Figure 6 are the transmission profiles for a typical fluorescence filter combination used in modern microscopes. The excitation filter spectrum (red curve) exhibits a high level of transmission (approximately 75 percent) between 450 and 490 nanometers with a center wavelength (CWL) of 470 nanometers. The dichromatic mirror (yellow curve) reflects wavelengths in the region of the excitation spectrum, while passing higher and lower wavelengths with relatively high efficiency. Note that zero percent transmission on the dichromatic mirror curve corresponds to 100 percent reflection. The pronounced dip in the transmission profile between 450 and 500 nanometers, which represents a peak in reflectance, serves to reflect the band of wavelengths passing from the excitation filter at a 90-degree angle and onto the specimen. The final component in the optical train, an emission or barrier filter (white curve), transmits wavelengths in the green visible light region, in the range between 520 and 560 nanometers. Boundaries between transmitted and reflected wavelength bands of the various superimposed spectra are designed to be as steep as possible to assure nearly complete separation of the reflected and transmitted wavelengths. A pattern of sinusoidally rising and falling spikes appearing in the dichromatic mirror spectrum is a common effect of the thin-film deposition process known as ringing. The performance of this filter combination is remarkable and is a clear demonstration of the rapid advances being achieved in thin film interference filter technology.
The filter nomenclature employed by Nikon derives from a mixture of terms dating back to the early 1990s. At that time, all of the Nikon complementary filter combinations were produced using the hard coat sputter technique, but many of the currently available filters take advantage of newer softer coating methods. Although soft coats are more susceptible to humidity and heat degradation, and must be handled more carefully than hard coat filters, they exhibit higher blocking value optical densities and provide greater ease of fine-tuning specific wavelength bands. Understanding the Nikon filter combination code nomenclature provides a mechanism to quickly determine whether a particular set will perform adequately for a specific fluorophore.
The first letter in the Nikon proprietary alphanumeric filter designation code indicates the wavelength excitation spectral region (for example, UV, V, B, and G, which are simple abbreviations for ultraviolet, violet, blue, and green, respectively). The number following the excitation code relates to the excitation filter passband width: 1 for narrow band excitation, 2 for medium and wide band excitation, and 3 for very wide band excitation. Finally, one or more letters following the excitation bandpass size number identifies the barrier filter characteristics. The code letter A indicates a standard longpass barrier filter with the lowest cut-on wavelength, while B designates a higher cut-on wavelength value for a longpass emission filter. Bandpass emission filters are identified with the letter E (referring to the term "enhanced") to indicate their superior performance with regard to eliminating crossover. The E/C filters are soft coat interference combinations designed for best performance with specific probes, such as DAPI, FITC, TRITC, and Texas Red.
The Fluorescence Light Budget
An estimation of the light fluxes in a typical fluorescence microscope is useful to outline constraints that will be encountered in producing digital images or during the visual observation of specimens. The excitation source is assumed, for this exercise, to be a standard 75-Watt xenon arc-discharge lamp having a mean luminous flux density of approximately 400 candelas per square millimeter (for other sources, see Table 1). When the lamp output is collected and directed through a 490-nanometer interference filter (having a 10-nanometer bandwidth and 75 percent transmission), about 2 milliWatts of light will pass through. After reflection by a 90-percent efficient dichromatic mirror, a light flux of 1.8 milliWatts enters the rear aperture of the microscope objective as the excitation beam.
With a 100x objective having a numerical aperture of 1.4, the area of the specimen illuminated will be 12 x 10 ¡Á E(-6) square centimeters, assuming a circular field of view about 40 micrometers in diameter. The light flux on the specimen is then about 150 Watts per square centimeter, which corresponds to a flux density of 3.6 x 10 ¡Á E(20) photons per square centimeter. Thus, the specimen illumination intensity is about 1000 times higher than that incident on the Earth¡¯s surface on a sunny day.
The fluorescence emission that results from the light flux discussed above depends on the absorption and emission characteristics of the fluorophore, its concentration in the specimen, and the optical path length of the specimen. In mathematical terms, the fluorescence produced (F) is given by the equation:
F = ¦Ò ¡Á Q ¡Á I
where ¦Ò is the molecular absorption cross-section, Q is the quantum yield, and I is the incident light flux (as calculated above). Assuming that fluorescein is the fluorophore, the absorption cross-section (¦Ò) is 3 x 10 ¡Á E(-16) square centimeters per molecule, Q equals 0.99, resulting in a value for F of 100,000 photons per second per molecule. If the dye concentration is 1 micromole per liter and is uniformly distributed in a 40-micrometer diameter disk with a thickness of 10 micrometers (volume equal to 12 picoliters), there are approximately 1.2 x 10 ¡Á E(-17) moles of dye or 7.2 million molecules in the optical path. If all of the molecules were excited simultaneously, the fluorescence emission rate would be 7.2 x 10 ¡Á E(11) photons per second (given the product of F and the number of dye molecules). The question of interest is how many of the emitted photons would be detected and for how long could this emission rate continue?
Luminous Density of Selected Light Sources
Lamp |
Current
(Amperes) |
Luminous Flux
(Lumens) |
Mean Luminous
Density (cd/mm2) |
Arc Size
(H x W)
(Millimeters) |
|
Mercury Arc
(100 Watt) |
5 |
2200 |
1700 |
0.25 x 0.25 |
|
Xenon Arc
(75 Watt) |
5.4 |
850 |
400 |
0.25 x 0.50 |
|
Xenon Arc
(500 Watt) |
30 |
9000 |
3500 |
0.30 x 0.30 |
|
Tungsten
Halogen |
8 |
2800 |
45 |
4.2 x 2.3 | |
|

|
|

|

|
Table 1
The efficiency of detection is a function of the optical collection efficiency and the detector quantum efficiency. A 1.4-numerical aperture objective with 100-percent transmission (an unrealistic condition) has a maximum collection efficiency, limited by the acceptance angle of about 30 percent. The transmission efficiency of the dichromatic mirror is 85 percent and that of the barrier filter is 80 percent. The overall collection efficiency is then about 20 percent or 140 billion photons per second. If the detector is a conventional charge-coupled device (CCD), the quantum efficiency is about 50 percent for the green fluorescein emission (at 525 nanometers), so the detected signal would be 70 billion photons per second or about 10 percent of the emitted fluorescence. Even with a perfect detector (100 percent quantum efficiency), only about 20 percent of the fluorescence emission photons can be detected.
The duration of fluorescence emission depends upon the rate of fluorophore destruction as a result of photobleaching. For fluorescein in an oxygenated saline solution, measurements indicate that each molecule can only emit about 36,000 photons before being destroyed. In a deoxygenated environment, the rate of photodestruction diminishes about tenfold, so 360,000 photons are produced per fluorescein molecule. The entire dye pool, in this example (7.2 million molecules), would then be capable of producing a minimum of 2.6 x 10 ¡Á E(11) and a maximum of 2.6 x 10 ¡Á E(12) photons. Assuming the emission rate of 100,000 photons per second per molecule calculated above, fluorescence could continue for only 0.3 to 3 seconds before photodestruction. In the case where 10 percent of the photon flux is detected, a signal of 7.2 x 10 ¡Á E(10) electrons per second would be obtained.
Following the argument of this example, if the detector is a 1000 x 1000 pixel CCD camera, this signal would be distributed over a million sensors, with approximately 72,000 electrons per sensor. For a scientific-grade CCD with 9-micrometer square sensors, the full well storage capacity is about 80,000 electrons and the read-out noise is less than 10 electrons. The signal-to-noise ratio would then be largely determined by photon statistical noise equal to the square root of the signal, approximately 268. In almost all cases, this high signal level could only continue for a very brief period of time before photodestruction occurs. The compromise utilized by most microscopists to prolong the observation period is a reduction in the incident light flux intensity so that only a fraction of the fluorophore molecules in the dye pool are excited and subjected to photodestruction. Thus, the signal-to-noise ratio rarely equals the theoretical maximum and typically ranges between 10 and 20 in fluorescence microscopy.
Detecting Single Molecules
Under ideal conditions, it is often possible to detect the fluorescence emission from a single molecule, provided that the optical background and detector noise are sufficiently low. As discussed above, a single fluorescein molecule could emit as many as 300,000 photons before it is destroyed by photobleaching. Assuming a 20-percent collection and detection efficiency, about 60,000 photons would be detected. Using avalanche photodiode or electron multiplying CCD detectors for these experiments, investigators have been able to monitor the behavior of single molecules for many seconds and even minutes. The major problem is adequate suppression of the optical background noise. Because many of the materials utilized in construction of microscope lenses and filters display some level of autofluorescence, efforts were initially directed toward the manufacture of very low fluorescence components. However, it soon became evident that fluorescence microscopy techniques utilizing total internal reflection (TIR) provided the desired combination of low background and high excitation light flux.
Total internal reflection fluorescence microscopy (TIRFM) takes advantage of the evanescent wave that is developed when light is totally internally reflected at the interface between two media having dissimilar refractive indices. The principle employing an external light source is illustrated in Figure 7(a). In this technique, a beam of light (usually an expanded laser beam) is directed through a prism of high refractive index, such as glass or sapphire, which abuts a lower refractive index medium of glass or aqueous solution. If the light is directed into the prism at higher than the critical angle, the beam will be totally internally reflected at the interface. The reflection phenomenon develops an evanescent wave at the interface by the generation of an electromagnetic field that permeates about 200 nanometers or less into the lower refractive index space. The light intensity in the evanescent wave is sufficiently high to excite the fluorophores within it, but because of its shallow depth, the volume excited is very small. The result is an extremely low-level background because so little of the specimen is exposed to the excitation light (only that portion within a 200-nanometer distance of the interface).
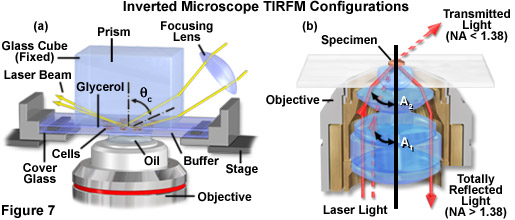
Total internal reflection fluorescence microscopy can also be conducted through a modification of the epi-illumination approached utilized in widefield techniques (as illustrated in Figure 7(b)). This method requires a very high numerical aperture objective (at least 1.4, but preferably 1.45 to 1.6) and partial illumination of the microscope field from one side by a small sport or more uniform illumination by a thin annulus. High refractive index lens immersion medium and microscope cover glass are required to achieve the illumination angle resulting in total internal reflection. As presented in Figure 7(b), light rays exiting the objective front lens element at an angle less than the critical angle (denoted as A(1)) in figure 7(b)) are transmitted away from the microscope. When the angle is increased to or beyond the critical angle (indicated a angle A(2) in Figure 7(b)), total internal reflection results.
Other popular advanced fluorescence techniques, such as fluorescence resonance energy transfer (FRET) and fluorescence recovery after photobleaching (FRAP), as well as spectroscopy, are often combined with total internal reflection to achieve additional information. The result is a very powerful tool for the study of individual fluorophores and fluorescently labeled molecules. The advantages resulting from the study of the properties of single molecules are only beginning to be appreciated. Thus, the current range of optical microscopy now extends from the single molecule to the entire animal.
Conclusions
The modern fluorescence microscope combines the power of high performance optical components with computerized control of the instrument and digital image acquisition to achieve a level of sophistication that far exceeds that of simple observation by the human eye. Microscopy now depends heavily on electronic imaging to rapidly acquire information at low light levels or at visually undetectable wavelengths. These technical improvements are not mere window dressing, but are essential components of the light microscope as a system.
The era when optical microscopy was purely a descriptive instrument or an intellectual toy is past. At present, optical image formation is only the first step toward data analysis. The microscope accomplishes this first step in conjunction with electronic detectors, image processors, and display devices that can be viewed as extensions of the imaging system. Computerized control of focus, stage position, optical components, shutters, filters, and detectors is in widespread use and enables experimental manipulations that were not humanly possible with mechanical microscopes. The increasing application of electro-optics in fluorescence microscopy has led to the development of optical tweezers capable of manipulating sub-cellular structures or particles, the imaging of single molecules, and a wide range of sophisticated spectroscopic applications. |





